Cytosolic retention of HtrA2 during mitochondrial protein import stress triggers the DELE1-HRI pathway
Paul Y Bi, Samuel A Killackey, Linus Schweizer, Damien Arnoult, Dana J Philpott, Stephen E Girardin
Gut microbiome modified by bariatric surgery improves insulin sensitivity and correlates with increased brown fat activity and energy expenditure
Jitender Yadav, Tao Liang, Tairan Qin, Nayanan Nathan, Katherine J.P. Schwenger, Lauren Pickel, Li Xie, Helena Lei, Daniel A. Winer, Heather Maughan, Susan J. Robertson, Minna Woo, Wendy Lou, Kate Banks, Timothy Jackson, Allan Okrainec, Susy S. Hota, Susan M. Poutanen, Hoon-Ki Sung, Johane P. Allard, Dana J. Philpott, Herbert Y. Gaisano
ATG16L1 protects from interferon-γ-induced cell death in the
small intestinal crypt
Elisabeth G. Foerster, Derek K.L. Tsang, Shawn Goyal, Susan J. Robertson, Lukian M. Robert, Heather Maughan, Catherine J. Streutker, Stephen E. Girardin, Dana J. Philpott
A single cell survey of the microbial impacts on the mouse small intestinal epithelium
Derek K.L. Tsang, Ryan J. Wang, Oliver De Sa, Arshad Ayyaz, Elisabeth G. Foerster, Giuliano Bayer, Shawn Goyal, Daniel Trcka, Bibaswan Ghoshal, Jeffrey L. Wrana, Stephen E. Girardin & Dana J. Philpott
Invading Bacterial Pathogens Activate Transcription Factor EB in Epithelial Cells through the Amino Acid Starvation Pathway of mTORC1 Inhibition
Liliane Cabral-Fernandes, Shawn Goyal, Armin Farahvash, Jessica Tsalikis, Dana J. Philpott, Stephen E. Girardin
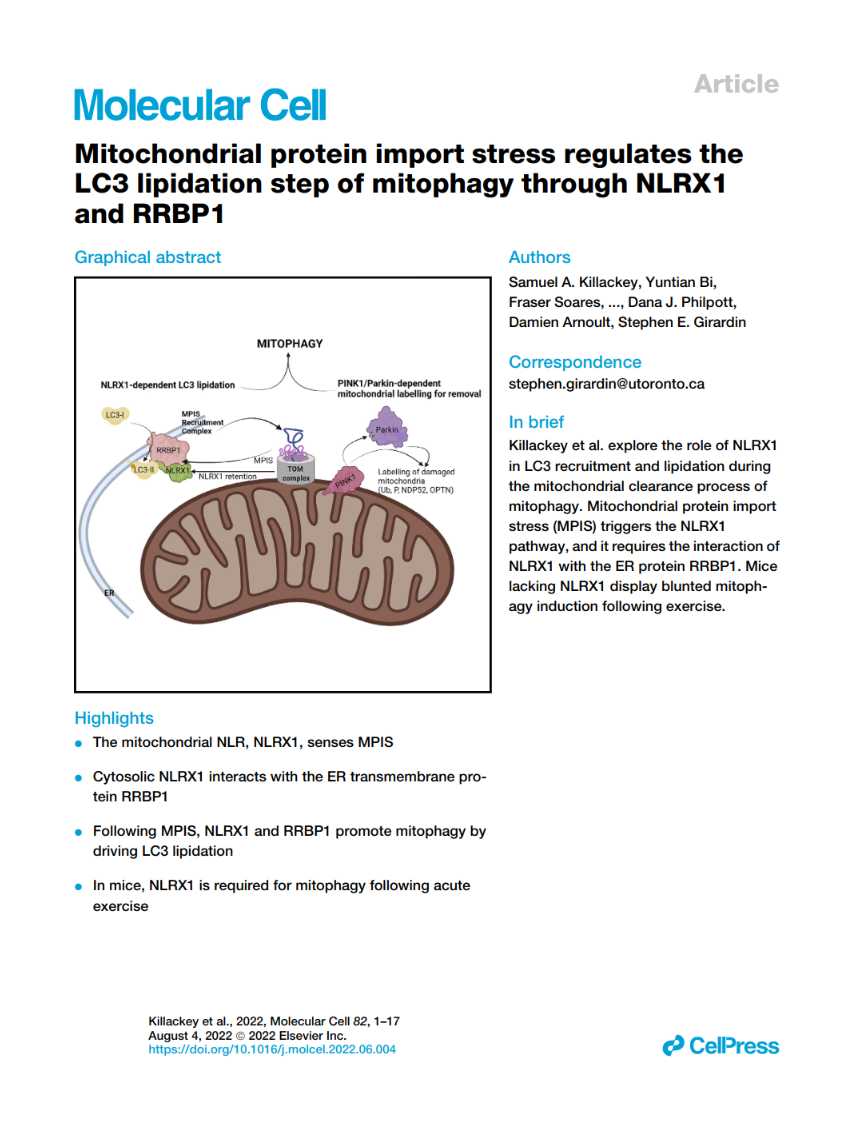
Mitochondrial protein import stress regulates the LC3 lipidation step of mitophagy through NLRX1 and RRBP1
Samuel A. Killackey, Yuntian Bi, Fraser Soares, Ikram Hammi, Nathaniel J. Winsor, Ali A. Abdul-Sater, Dana J. Philpott, Damien Arnoult, Stephen E. Girardin
Here we showed that the underlying trigger for the LC3 lipidation step on membranes adjacant to mitochondria is the defective import of proteins into mitochondria, coined as mitochondrial protein import stress (MPIS). During MPIS, the import into mitochondria of the Nod-like receptor (NLR) protein NLRX1 is prevented and, upon cytosolic retention, NLRX1 forms a complex with the endoplasmic reticulum (ER) protein RRBP1, which is required for local lipidation of LC3. During MPIS, RRBP1 is recruited at the vicinity of mitochondria independently of NLRX1, and we propose that this recruitment may initially serve a homeostatic function, to help restoring efficient translation of mitochondria-targetting proteins. We speculate that the dual requirement for NLRX1 and RRBP1 to trigger mitophagy may ensure that mitophagy will only ensue when the mitochondrial protein import machinery is obstructed (leading to NLRX1 cytosolic accumulation) and when the translation machinery does not work optimally (leading to the loss of RRBP1-ribosome contacts and protein redistribution).
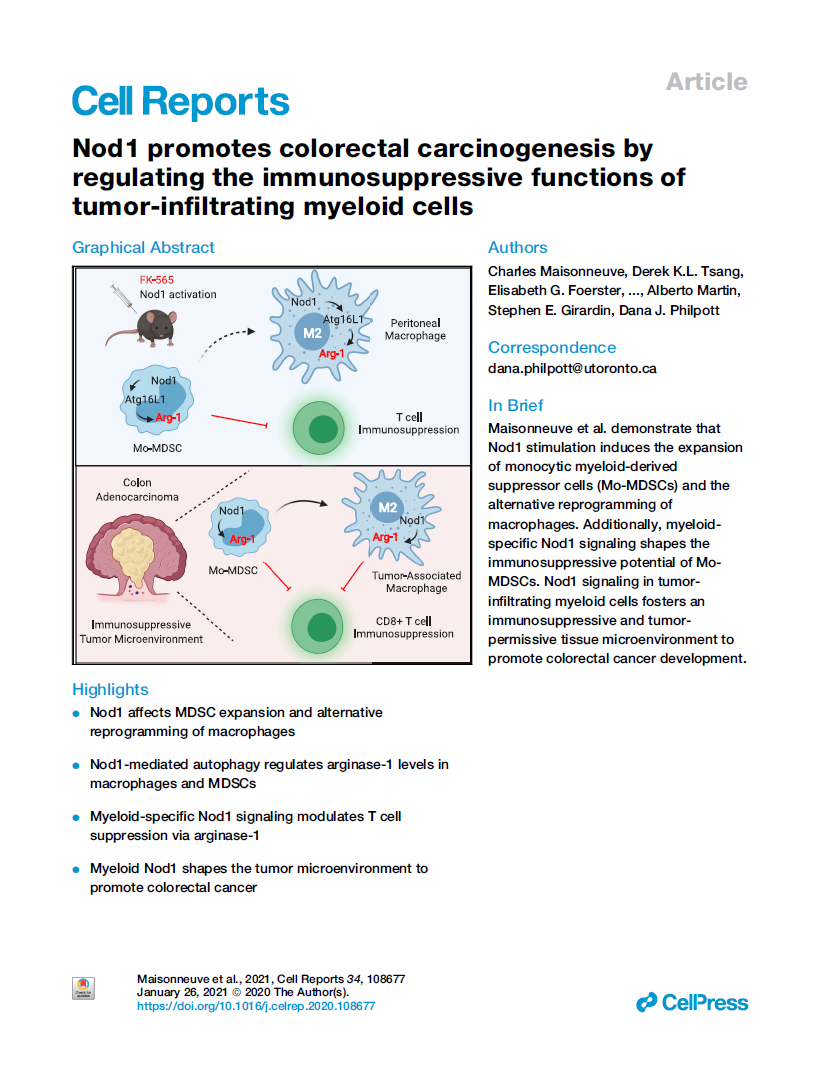
Nod1 promotes colorectal carcinogenesis by regulating the immunosuppressive functions of tumor-infiltrating myeloid cells
Charles Maisonneuve, Derek K.L. Tsang, Elisabeth G. Foerster, Lukian Maxence Robert, Tapas Mukherjee, Dave Prescott, Ivan Tattoli, Paul Lemire, Daniel A. Winer, Shawn Winer, Catherine J. Streutker, Kaoru Geddes, Ken Cadwell, Richard L. Ferrero, Alberto Martin, Stephen E. Girardin, Dana J. Philpott
Here we revisited the potential role of the bacterial peptidoglycan sensor Nod1 in controlling colorectal cancer. We first show that Nod1 peptidoglycan ligand promotes an autophagy-dependent reprogramming of macrophages toward an alternative phenotype. Moreover, we found that Nod1 stimulation induces the expansion of myeloid-derived suppressor cells (MDSCs) and maintains their immunosuppressive potential via arginase-1 activity. Furthermore, we show here that myeloid-intrinsic Nod1 expression sustains intra-tumoral arginase-1 levels to foster an immunosuppressive and tumor-permissive microenvironment during colorectal cancer (CRC) development.
The eIF2α kinase HRI triggers the autophagic clearance of
cytosolic protein aggregates
Tapas Mukherjee , Valeria Ramaglia, Mena Abdel-Nour, Athanasia A Bianchi, Jessica Tsalikis, Hien N Chau, Suneil K Kalia, Lorraine V Kalia, Jane-Jane Chen, Damien Arnoult, Jennifer L Gommerman , Dana J Philpott, Stephen E Girardin
Here, we investigated the role of HRI in proteotoxic stress and demonstrated that HRI-dependent signaling is critical for the clearance of protein aggregates through autophagy. We also present evidence that the HRI pathway can prevent accumulation of toxic alpha-synuclein aggregates, in vitro and in the mouse central nervous system.
Mitophagy pathways in health and disease
Samuel A Killackey, Dana J Philpott, Stephen E Girardin
This review article provides a comprehensive description of the molecular mechanisms associated with the process of mitochondrial autophagy (=mitophagy), and presents an overview of the physiological and disease conditions associated with mitophagy
NOD2 modulates immune tolerance via the GM-CSF–dependent generation of CD103+ dendritic cells
David Prescott, Charles Maisonneuve, Jitender Yadav, Stephen J. Rubino, Stephen E. Girardin, and Dana J. Philpott
Here, we demonstrate a function of NOD2, whereby its activation leads to the generation of immunological tolerance to foreign antigens by inducing tolerant dendritic cells both systemically and within the intestine. This occurs via the generation of the cytokine GM-CSF, a factor that itself has previously been linked to Crohn’s disease development. The identification of a common immunological pathway shared by these two factors will open up new avenues for therapy and further research to identify the precise etiology of this chronic, debilitating condition.
The heme-regulated inhibitor is a cytosolic sensor of protein misfolding that controls innate immune signaling
Mena Abdel-Nour, Leticia A. M. Carneiro, Jeffrey Downey, Jessica Tsalikis, Ahmed Outlioua, Dave Prescott, Leandro Silva Da Costa, Elise S. Hovingh, Armin Farahvash, Ryan G. Gaudet, Raphael Molinaro, Rob van Dalen, Charles C. Y. Lau, Farshad C. Azimi, Nichole K. Escalante, Aaron Trotman-Grant, Jeffrey E. Lee, Scott D. Gray-Owen, Maziar Divangahi, Jane-Jane Chen, Dana J. Philpott, Damien Arnoult*, Stephen E. Girardin*
This publication revealed a new mechanism by which the assembly of innate immune sensors, including NOD2 and NOD1, is regulated by the integrated stress response pathway, highlighting a novel link between cellular stress and innate immunity.
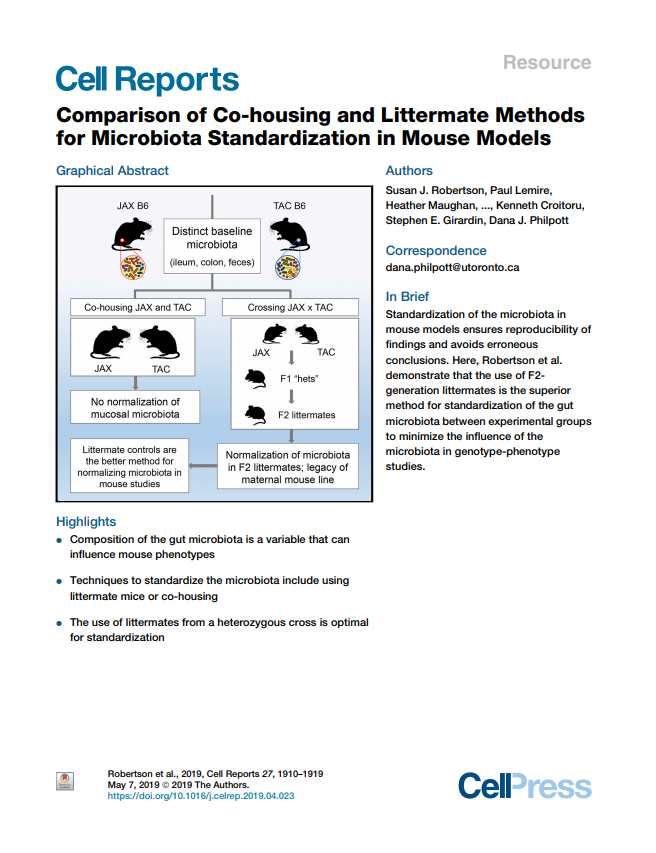
Comparison of co-housing and littermate methods for microbiota standardization in mouse models
Susan J. Robertson, Paul Lemire, Heather Maughan, Ashleigh Goethel, Williams Turpin, Larbi Bedrani, David S. Guttman, Kenneth Croitoru, Stephen E. Girardin, Dana J. Philpott
This publication establishes guidelines on microbiota analyses in mouse models, in particular when comparing two lines of mice (such as wild type and knockout animals).
Trace levels of peptidoglycan in serum underlie the NOD-dependent cytokine response to endoplasmic reticulum stress
Raphael Molinaro, Tapas Mukherjee, Robert Flick, Dana J. Philpott and Stephen E. Girardin
Here we revealed that trace amounts of the bacterial cell wall molecule peptidoglycan can be found in serum, which can have important physiological implications for homeostatic regulation of NOD-dependent signaling. Moreover, as we show in this publication, the presence of peptidoglycan in serum can also drive NOD-dependent induction of pro-inflammatory signaling in response to Ca2+-dependent signals.
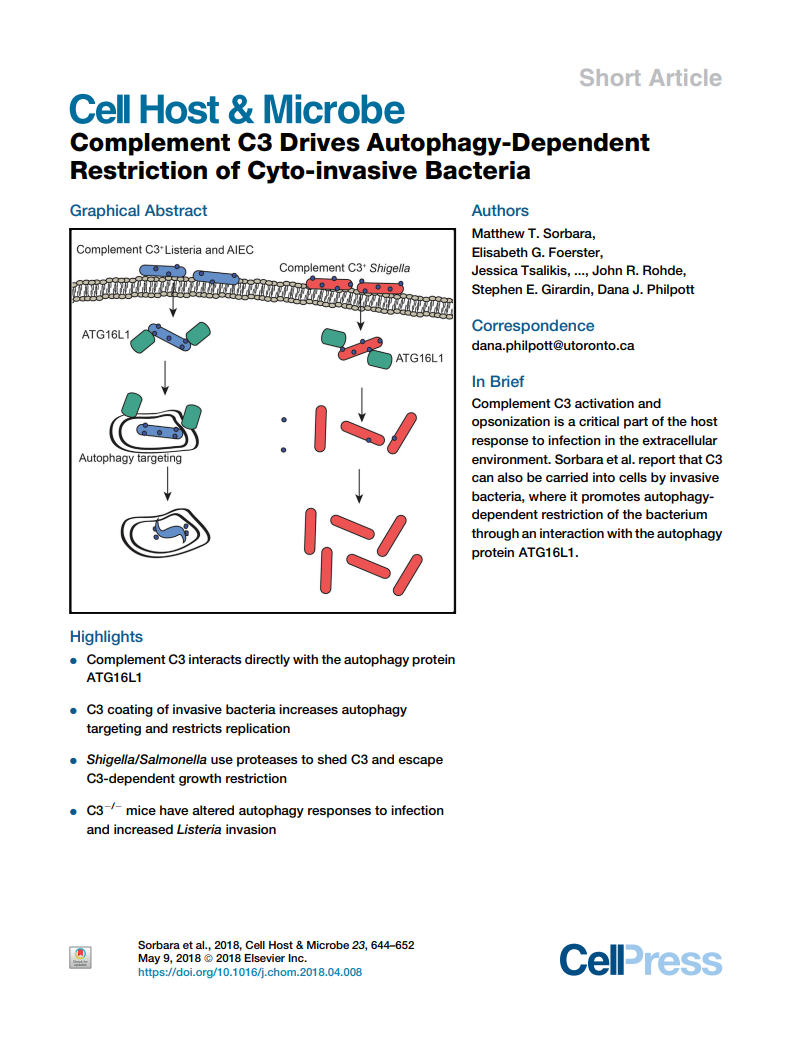
Complement C3 drives autophagy-dependent restriction of cyto-invasive bacteria
Matthew T. Sorbara, Elisabeth G. Foerster, Jessica Tsalikis, Mena Abdel-Nour, Joseph Mangiapane, Imogen Sirluck-Schroeder, Ivan Tattoli, Rob van Dalen, David E. Isenman, John R. Rohde, Stephen E. Girardin, and Dana J. Philpott
Here we present the unexpected finding that the complement protein C3, which coats bacterial cell surfaces for innate immune clearance, also serves as a molecular binding partner for ATG16L1, a key protein involved in autophagy processes whose gene is an important susceptibility gene for Crohn’s disease.
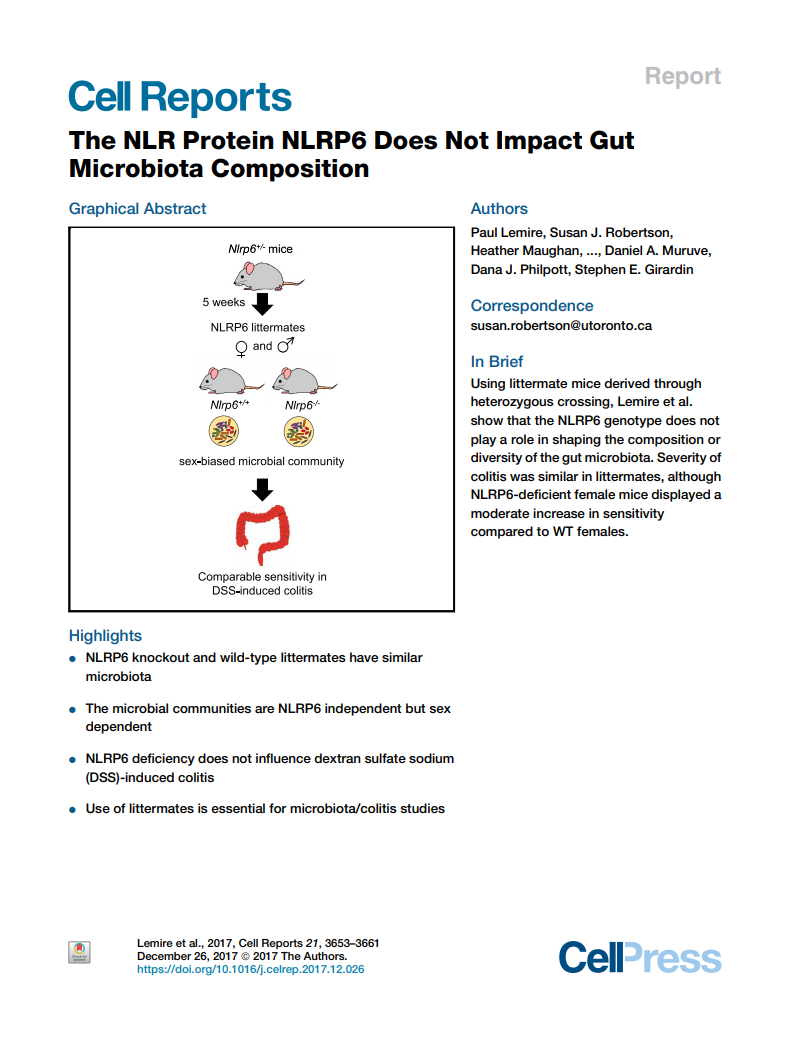
The NLR protein NLRP6 does not impact gut microbiota composition
P Lemire, SJ Robertson, H Maughan, I Tattoli, CJ Streutker, JM Platnich, DA Muruve, DJ Philpott and SE Girardin
Here we revisited the previously reported role of NLRP6 in shaping the intestinal microbiota using experiments performed in littermate animals.
NLRX1 acts as an epithelial-intrinsic tumour suppressor through the modulation of TNF-mediated proliferation
I Tattoli, SA Killackey, EG Foerster, R Molinaro, C Maisonneuve, MA Rahman, S Winer, DA Winer, CJ Streutker, DJ Philpott and SE Girardin
We showed a key role for the NLR protein NLRX1 in epithelial regeneration following injury, and how this function directly impacts on colon cancer.
Intracellular bacterial pathogens trigger the formation of U bodies through metabolic stress induction
J Tsalikis, I Tattoli, A Ling, MT Sorbara, DO Croitoru, DJ Philpott and SE Girardin
In this article, we used cellular models of infection similar to those proposed in the present project to demonstrate that intracellular bacterial pathogens strikingly perturb the homeostasis of U snRNP maturation through the induction of cellular stress responses. We further demonstrated how this impacted on the cellular splicing machinery.
The protein ATG16L1 suppresses inflammatory cytokines induced by the intracellular sensors Nod1 and Nod2 in an autophagy-independent manner
MT Sorbara, LK Ellison, M Ramjeet, LH Travassos, NL Jones, SE Girardin* and DJ Philpott*
In this publication, we analyzed the functional interplay between two proteins, Nod2 and Atg16l1, whose genes are mutated in Crohn’s disease. Unexpectedly, we found that the autophagy protein Atg16l1 modulated innate immune defense signaling independently from autophagy. These results change the paradigm of the importance of autophagy regulation in Crohn’s disease.
Listeria phospholipases subvert host autophagic defenses by stalling pre-autophagosomal structures
I Tattoli, MT Sorbara, C Yang, SA Tooze, DJ Philpott and SE Girardin
This publication shows for the first time that Listeria uses two toxins (Phospholipases A and B) to actively suppress the autophagy-associated mechanism of host defense in infected cells. This study opens up the possibility that other pathogens could also use a similar strategy to escape innate immunity.
Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program
I Tattoli, M Sorbara, D Vuckovic, A Ling, F Soares, LAM Carneiro, C Yang, A Emili, DJ Philpott and SE Girardin
This publication presents the first demonstration that bacterial invasion results in an acute phase of amino acid starvation in infected host cells. This observation links bacterial infection to metabolic stress pathways and explains how autophagy is turned on in infected cells.
Nod1 and Nod2 activation in the stromal compartment instruct dendritic cells to initiate Th2 immunity
JG Magalhaes, SJ Rubino, LH Travassos, L LeBourhis, W Duan, G Sellge, K Geddes, C Reardon, M Lechmann, LA Carneiro, T Selvanantham, JH Fritz, BC Taylor, D Artis, TW Mak, M Croft, SE Girardin and DJ Philpott
This publication shows, using bone-marrow chimeric mice, that Nod1 and Nod2 expression within the stromal compartment is necessary for priming of effector CD4(+) Th2 responses and specific IgG1 antibodies. In contrast, sensing of these ligands by dendritic cells was not sufficient to induce Th2 immunity, although these cells contribute to the response. We also found that full Th2 induction upon Nod1 and Nod2 activation was dependent on both TSLP production by the stromal cells and up-regulation of the costimulatory molecule OX40 ligand on dendritic cells. This study provides in vivo evidence of how systemic Th2 immunity is induced in the context of Nod stimulation.
Identification of an innate Th17 response to intestinal bacterial pathogens
K Geddes, SJ Rubino, JG Magalhaes, C Streutker, L Le Bourhis, JH Cho, S Robertson, CJ Kim, R Kaul, DJ Philpott and SE Girardin
This publication identified a key role for the NLR proteins Nod1 and Nod2 in the control of early Th17 immune responses in the intestine following infection with enteric bacterial pathogens. Because both Nod-dependent signaling and Th17 pathways are associated with Crohn’s disease, these findings provide novel insights into the pathogenesis of this disease. This article was the subject of a Podcast interview in the June 2011 issue of Nature Medicine.
Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the site of bacterial entry
LH Travassos, LAM Carneiro, M Ramjeet, S Hussey, Y-G Kim, L Yuan, F Soares, JG Magalhães, L Le Bourhis, IG Boneca, A Allaoui, NL Jones, G Nuñez, SE Girardin, and DJ Philpott
This publication reports that the NLR proteins Nod1 and Nod2 participate in the targeting of autophagy proteins to the site of bacterial entry, thereby possibly influencing autophagy-dependent control of the infection. Because polymorphisms in both Nod2 and ATG16L1 have been shown to confer susceptibility to Crohn’s disease, these results highlight the importance of the Nod/autophagy axis in the development of this inflammatory disorder.
Shigella induces mitochondrial dysfunction and cell death in nonmyeloid cells
LAM Carneiro, LH Travassos, F Soares, I Tattoli, JG Magalhaes, MT Bozza, MC Plotkowski, PJ Sansonetti, JD Molkentin, DJ Philpott and SE Girardin
This publication identifies the mechanism through which the intracellular bacterium Shigella induces cell death in non-myeloid cells. In particular, the key impact of infection on mitochondrial dysfunction was uncovered.
NLRC5 limits the activation of inflammatory pathways
S Benko, JG Magalhaes, DJ Philpott and SE Girardin
In this publication, we demonstrate that NLRC5 plays a key role in controlling inflammatory pathways in murine macrophages, in part through the specific regulation of IL-10 expression in response to inflammatory cues.
An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix
D Arnoult, F Soares, I Tattoli, C Castanier, DJ Philpott and SE Girardin
Here we demonstrate that NLRX1 is a mitochondrial protein that resides in the matrix of this organelle at steady state, where it can interact with UQCRC2, a protein of the electron transport chain.
pH-dependent internalization of muramyl peptides from early endosomes enables Nod1 and Nod2 signaling
J Lee, I Tattoli, KA Wojtal, SR Vavricka, DJ Philpott and SE Girardin This
This publication provides evidence that muramyl peptides, which are ligands of NOD proteins, are internalized into host cells through clathrin-dependent endocytosis, and can be brought into the cytosol via SLC15A4, an oligopeptide transporter.
Nod2-dependent Th2 polarization of antigen-specific immunity
JG Magalhaes, JH Fritz, L Le Bourhis, G Sellge, LH Travassos, T Selvanantham, SE Girardin, JL Gommerman and DJ Philpott
Here we studied how stimulation of NOD2 by MDP can drive antigen-dependent adaptive immune responses in vivo.
Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity
JH Fritz, L Le Bourhis, H Fsihi, G Sellge, JG Magalhaes, H Fsihi, TA Küfer, C Collins, J Viala, RL Ferrero, SE Girardin and DJ Philpott
This publication characterized the role played by Nod1, in hematopoietic and non- hematopoietic cells, in driving antigen-dependent adaptive immune responses to DAP-type peptidoglycan.
NLRX1 is a mitochondrial NOD-like receptor that amplifies NF-kB and JNK pathways by inducing reactive oxygen species production
I Tattoli, LA Carneiro, M Jéhanno, JG Magalhaes, Y Shu, DJ Philpott, D Arnoult and SE Girardin
This represents the first demonstration that the NLR protein NLRX1 is a mitochondrial protein.
A critical role for peptidoglycan N-deacetylation in Listeria evasion from host innate immune response
IG Boneca, O Dussurget, D Cabanes, MA Nahori, S Sousa, M Lecuit, E Psylinakis, V Bouriotis, JP Hugot, A Coyle, J Bertin, A Namane, JC Rousselle, N Cayet, MC Prevost, V Balloy, M Chignard, DJ Philpott, P Cossart and SE Girardin
This publication shows for the first time that peptidoglycan modifications can serve as a virulence strategy for bacterial pathogens, such as Listeria. This study opens up the possibility that other pathogens could also use this strategy to escape innate immunity.
Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2
SE Girardin, Travassos L.H., Hervé M., Blanot D., Boneca I.G., DJ Philpott, Sansonetti P.J., Mengin-Lecreulx D.
This publication reports for the first time the characterization of the detailed structure requirements of the muropeptides detected by both Nod1 and Nod2. This work has put forward the new concept that peptidoglycan-modifying enzymes could represent virulence factors in some pathogenic bacteria, a theme that is now being studied by several laboratories over the world.
Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan
SE Girardin, Boneca IG, Carneiro LAM, Antignac A, Jéhanno M, Viala J, Tedin K, Taha MH, Labigne A, Zähringer U, Coyle AJ, DiStefano PJ, Bertin J, Sansonetti PJ, and DJ Philpott
This was the first publication to show that the Nod-like receptor (NLR) protein Nod1 is a pattern recognition molecule that senses a fragment of bacterial peptidoglycan.
Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection
SE Girardin, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, DJ Philpott, Sansonetti PJ.
In this study we demonstrated that Nod2, which is implicated in the development of Crohn's disease, recognizes a fragment of bacterial peptidoglycan called muramyl dipeptide. These findings support the notion that the pathogenesis of Crohn's disease stems from a defect in host-bacteria interactions.
CARD4/Nod1 mediates NF-κB and JNK activation by invasive Shigella flexneri
Stephen E. Girardin, Régis Tournebize, Maria Mavris, Anne-Laure Page, Xiaoxia Li, George R. Stark, JohnBertin, Peter S. DiStefano, Moshe Yaniv, Philippe J. Sansonetti & Dana J. Philpott
Here we report the first characterization of a Nod-like receptor (NLR) protein, Nod1, as an intracellular sensor of bacterial infection. While we initially reported that Nod1 could detect bacterial LPS, we later corrected this statement by showing that Nod1 actually detects a common contaminant of LPS, DAP-type peptidoglycan (see Girardin et al, Science 2003).